Photonics and Plasmonics Nanoresearch Laboratory
Welcome!
The research interests of our group evolve around the development of optically active nanostructures such as plasmonic nanoparticles for different applications, ranging from the detection of biomolecules to solar energy conversion. Plasmonic nanoparticles can strongly enhance local electromagnetic field; and their ability to concentrate light in the nanoscale have found a broad range of applications. Presently, our research group focus on two specific areas: (1) Development of efficient photothermal materials which can convert light into heat for different applications such as solar mediated catalysis, solar enhanced water evaporation and photothermal recycling of polymer. (1) Development of plasmonic materials to efficiently concentrate light in the nanoscale to drive energy-expensive reactions. Our group uses both experimental and theoretical tools to gain a fundamental understanding of the optical and electronic properties of different plasmonic nanoparticles. We particularly focus on the development of alternative plasmonic nanomaterials which can replace conventional plasmonic materials such as gold and silver. We are studying refractory plasmonic nanomaterials such as titanium nitride nanomaterials for efficient conversion of light into other forms of energies such as thermal, chemical, and electrical energy. Additionally, we study bimetallic plasmonic nanoparticles. The possibility to control and tune optical properties of bimetallic plasmonic materials assumes a paramount relevance in the development of different applications such as plasmon enhanced catalysis as well as polymer recycling and additive manufacturing.
Research Highlights
Refractory Plasmonic Transition Metal Nitride Nanoparticles as Support for Transition Metal Catalysts
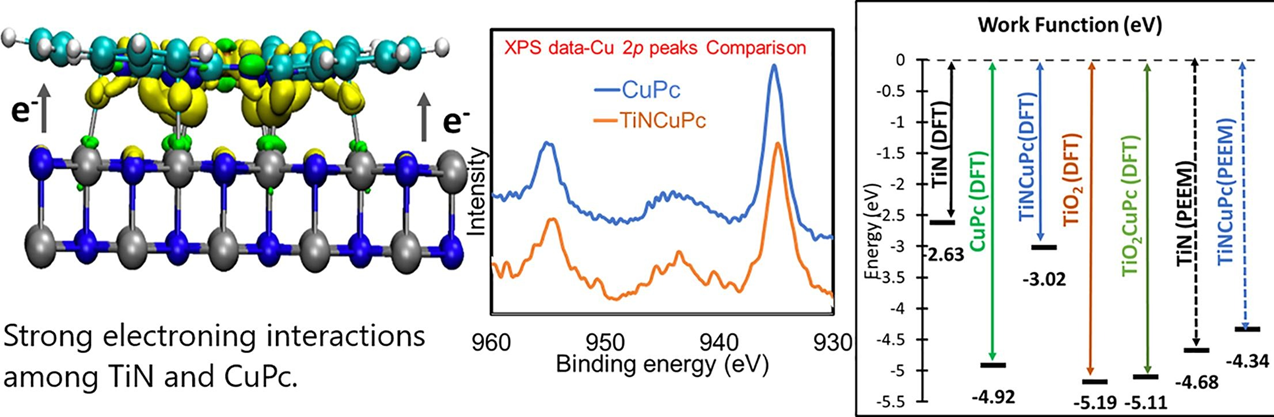
We revealed a strong electronic interactions between transition metal phthalocyanine (TMPc’s) on a refractory transition metal nitride support, specifically copper phthalocyanine (CuPc) on titanium nitride (TiN). X-ray Photoelectron Spectroscopy (XPS), photoelectron emission microscopy (PEEM) and density functional theory (DFT) calculations are presented to support our observation. Interestngly, presence of a few nanometer native oxide layer on the surface of the TiN nanoparticles, which consists of TiN, TiO2, and Titanium oxynitrides (TixOyNz) does not influence the nature of charge transfer among TiN and CuPc. Substantial deviations are however found between photoelectron emission microscopy (PEEM) measured work function for TiN (4.68 eV) and theoretically calculated work function for pristine stoichiometric TiN (2.63 eV) due to the presence of an oxide layer on the TiN surface. Our studies open up an opportunity to apply a new class of materials based on transition metal phthalocyanine/transition metal nitride composites to catalysis and optoelectronic devices. (Applied Surface Science 614 (2023) 156204)
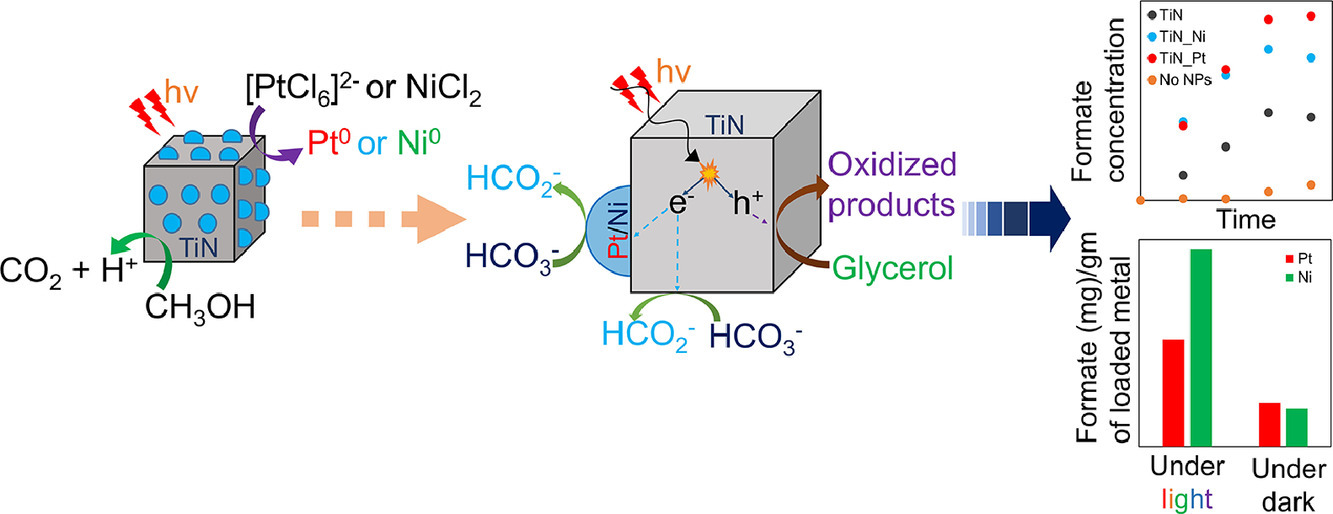
We have successfully deposited transition metal catalyst Nickel and Platinum on refractory plasmonic titanium nitride (TiN) nanoparticles using visible light induced plasmon enhanced deposition method. The catalytic properties of the transition metal catalysts on TiN supports were demonstrated using the visible light-mediated bicarbonate to formate reduction reaction in the presence of glycerol as the hole-scavenger. The findings of this study can be used to synthesize stable and efficient visible light-responsive plasmonic composite catalysts using mild reaction conditions, which can accelerate different energy extensive reactions using light. (Materials Research Bulletin 152 (2022) 111834)
Refractory plasmonic nanomaterials assisted recyling of thermosets
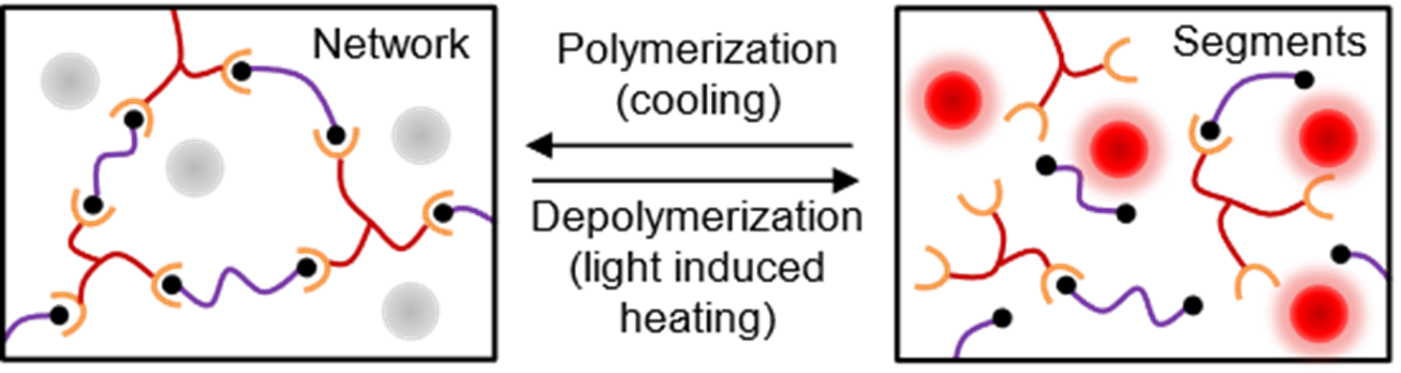
Thermosetting polymers such as epoxies are an important class of polymers which are
extensively used for many applications such as structural materials, electronics packaging,
and adhesives. These thermosetting polymers are known for their stable thermomechanical
properties and excellent chemical resistance. However, these thermosetting polymeric
materials are intrinsically not recyclable because of their irreversible cross-linked
network structure. Our research group is working on developing recyclable thermoset
polymers which can be recycled through depolymerization using solar light easily and
energy efficiently. To accomplish this we are developing thermo-reversible epoxy/
refractory plasmonic titanium nitride composites. The well-dispersed plasmonic nanomaterials
in the epoxy matrix can act as a localized thermal source that convert light into
localized heat efficiently rapidly to trigger the depolymerization. (Recently accepted
manuscript, 2022, ACS Applied Polymer Materials)
Relaxation Dynamics Studies of Plasmonic Composites
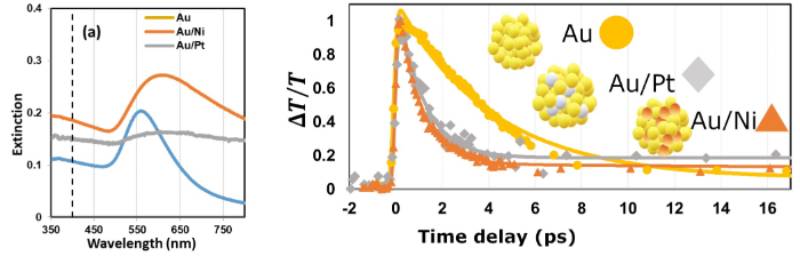
We studied optical properties of bimetallic nanoparticles comprised of plasmonic nanoparticle such as gold and catalyst such as nickel and platinum. We applied ultrafast time-resolved spectroscopy to examine the rate of different plasmon dephasing pathways, such as electron-electron scattering, electron-phonon coupling, and phonon-phonon scattering. We found the light absorption and relaxation dynamics of the bimetallic plasmonic catalysts can be tuned by tuning their composition. Developing photothermal catalysts combining plasmonic nanoparticles with catalytic metals extends the benefits of plasmonic nanoparticles to any energy-extensive industrial processes accelerated by those catalysts. (Nanoscale, 2020,12, 10284-10291)
Plasmon enhanced catalysis
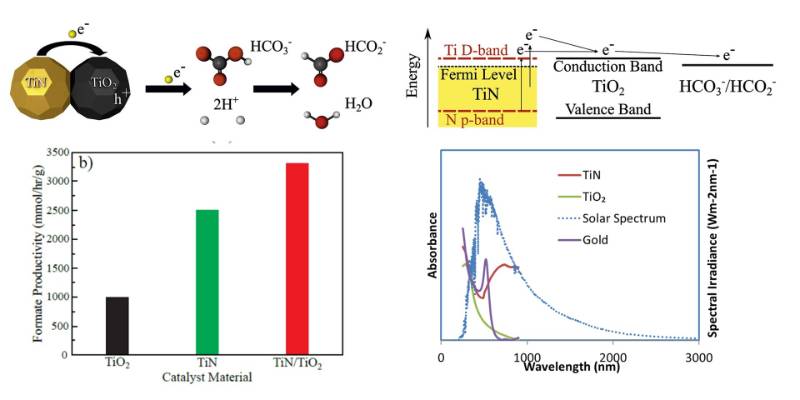
The high temperature stability, strong surface plasmon resonance and the ability to absorb broad spectrum of visible to near infrared light of transition metal nitrides such as titanium nitride (TiN) motivated us to study these materials for photocatalytic reactions. We showed an efficient photocatalytic reduction of bicarbonate on TiN and TiO2/TiN composite nanocatalysts under solar light illumination. TiN nanoparticles, in combination with titanium dioxide (TiO2) nanostructures significantly enhance the photoreduction of bicarbonate to produce formate when glycerol is used as a hole scavenger. Interestingly, we found that TiN nanoparticles alone can also behave as an excellent plasmonic catalyst. Moreover, TiN nanoparticles remain stable under reaction conditions (high pH) for extended periods of solar light exposure re (8 hours). (Solar Energy Materials and Solar Cells, 2019,200, 109967)
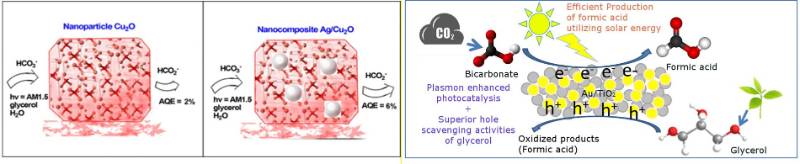
The strong electromagnetic field at the surface of plasmonic nanoparticles such as gold and silver are utilized to manipulate the energy and electron absorption of vicinal photocatalyst such as TiO2 or copper(I) oxide. We reported the efficient production of formic acid through simultaneous photoreduction of bicarbonate and oxidation of glycerol in the presence of a composite catalyst comprised of the plasmonic and the photocatalyst nanoparticles. (Journal of CO2 Utilization, 2017,22, 117-123; ACS Sustainable Chemistry & Engineering, 2018, 6, 1872-1880)
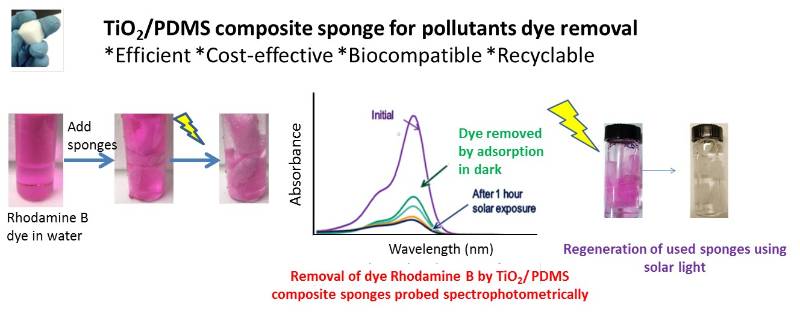
PDMS sponges are suitable substrate for selective absorption of oils and organic solvents from water and can be elastically deformed into any shape and can be compressed repeatedly in air or liquids without collapsing enabling excellent recyclability. We enhanced those sponges by incorporating nanocatalysts making them suitable for photocatalytic and antibacterial activities, thus promoting potential in environmental applications. (Journal of water process engineering, 2018, 24, 74-82)
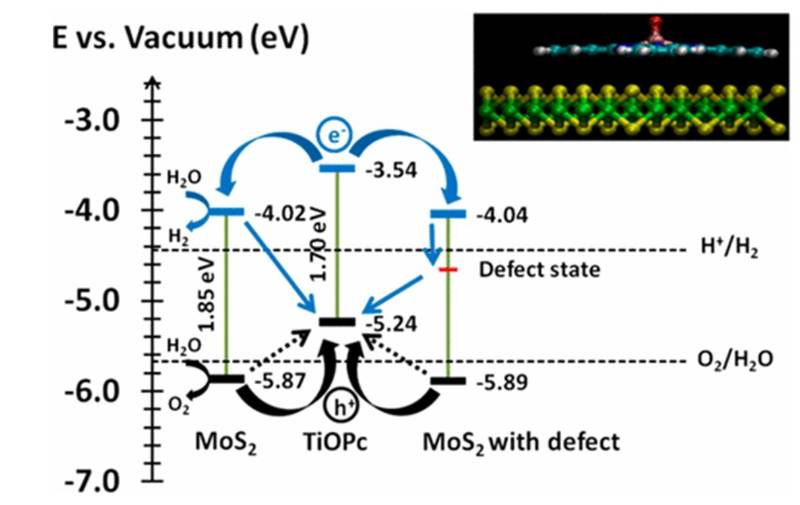
Two-dimensional layered nanomaterials such as molybdenum disulphide (MoS2) nanomaterials are optically active with potential applications in photocatalysis. We manipulated the electronic and optical properties of monolayer molybdenum disulfide by nonsubstitutional doping with macrocyclic organic metallic molecules like titanyl phthalocyanine (TiOPc) or copper phthalocyanine (CuPc). The implication of the results is discussed in term of their applicability in important reactions like hydrogen evolution by water dissociation. (J. Phys. Chem. C 2017, 121, 5, 2959–2967)
Funding and other resources

 Private Industry
Private Industry